Class 11 Chemistry
Redox Reactions

Electrochemical cell
- Electrochemical cell is the cell in which chemical energy gets converted to electric energy.
- In it indirect redox reactions takes place.
- These reactions are spontaneous that is free energy change for this reaction is negative.
- This cell consists of two half cells.
- In one half cell , there is a aqueous 1molar Zinc sulphate solution with Zinc rod dipped in it.
- In other half cell, there is a 1 molar aqueous solution of Copper sulphate solution with Copper rod dipped in it.
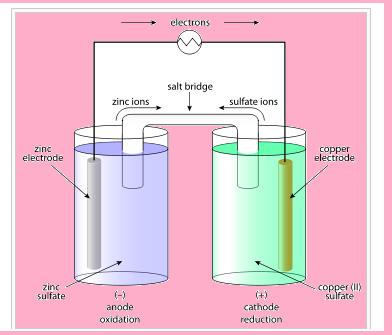
- These electrodes by means of wire are attached to galvanometer.
- A U-shaped tube is taken, which is sealed from both the ends with cotton plug.
- In this, the electrolyte that is inert electrolyte is taken like Potassium nitrate, Ammonium nitrate etc. The electrolyte present is in semi-liquid state.
Observations
- With time we see that Zinc rod loses weight, as it has more tendency to loose electrons that is:
Zn -2 electrons --> Zn2+ (Oxidation)
Zinc Zinc Ion
- These electrons released by zinc, travel to another beaker by means of wire. In doing so, they cause deflection in galvanometer and produce current. This current travel in the direction opposite to the flow of electrons.
- These electrons move to another half cell, where copper ions gain these electrons that is reduction occur. As a result, copper metal start depositing on electrode. The reaction that occurs is shown below:
Cu2+ + 2electrons --> Cu(reduction)
Copper ions Copper Metal
Functions of salt bridge
- It connects the circuit internally by connecting the solutions.
- It helps in maintain neutrality.
With passage of time, the left container will have excess positive charge around electrode. Due to which further oxidation stops .Whereas in other beaker negative charge will exceeds, which will start repelling electrons. Therefore, at that time salt bridge comes into action. The oppositely charged electrolyte ions start diffusing into half cells in order to neutralize the excess charge. Hence, the cell keeps on working.
The electrolyte that is selected must fulfill two conditions:
- Size of its cation and anion should be equal.
- Electrolyte in salt bridge should not interact with the main electrolyte of half cells.
The overall reaction that takes place is:
Zn + Cu2+ --> Zn2+ + Cu
Zinc Copper Zinc Ion Copper Metal
Representation of the cell
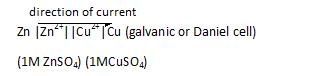
Formula to calculate standard electrode potential of cell (when concentration of electrolyte is 1 molar)
E=Ec-Ea (where ‘c’ is cathode and ‘a’ is anode).
Class 11 Chemistry Redox Reactions NCERT Chapter 7 Free Notes for Best Revision
Revision of Class 11 Chemistry Redox Reactions is a crucial aspect of effective learning. Revision plays a vital role in the learning process and is especially important before exams. Here are some key points you can consider emphasizing in your content:
- Retention and Memory: Regularly reviewing and revisiting the material of Class 11 Chemistry Redox Reactions helps reinforce the concepts in students' minds. It strengthens memory pathways, making it easier to recall information during exams and beyond.
- Consolidation of Knowledge: When you revise notes, you are essentially consolidating your knowledge. This means connecting new information with what you already know, making the overall understanding more robust.
- Identifying Knowledge Gaps: Revision allows students to identify any gaps in their understanding or areas where they need further clarification. This gives you a chance to seek help or delve deeper into those topics. For detailed understanding, you can always refer to the videos of Redox Reactions Class 11 Chemistry NCERT Chapter 7 on LearnoHub.com
- Building Confidence: As you revise Redox Reactions Class 11 Chemistry and become more familiar with the content, your confidence in your abilities grows. Confidence is a crucial factor in exam performance as it reduces anxiety and allows you to approach exams with a positive mindset.
- Different Revision Techniques: Use a variety of revision techniques such as summarizing notes, creating flashcards, practicing past papers, discussing concepts with peers, and teaching others. Different techniques work for different students, and it's essential to find what suits you the best. You can also attend the LIVE Revision classes on LearnoHub.com or watch the LIVE Revision Race videos of LearnoHub on Redox Reactions Class 11 Chemistry NCERT Chapter 7.
- Spacing Effect: Spacing out revision sessions over time, rather than cramming all at once, has been shown to improve long-term retention. Create a revision schedule leading up to the exams to allow for spaced practice.
- Regular Revision over Cramming: Regular and consistent revision throughout the academic year is very important. Waiting until the last moment to cram everything can be overwhelming and less effective than spaced-out revision.
- Self-Assessment: Assess your understanding periodically through quizzes or self-tests. This helps you to gauge your progress and identify areas that need further attention. Refer Class 11 Redox Reactions Online Tests.
- Balanced Approach: Remind students to strike a balance between revision and other activities. Adequate rest, exercise, and relaxation are essential for optimal learning and performance.
- Seeking Help: If you face difficulties during the revision process, Refer the videos of Class 11 Chemistry Redox Reactions. Clearing doubts promptly is crucial for a better grasp of the subject matter. You can always ask your doubts on Redox Reactions Class 11 Chemistry NCERT Chapter 7. “Ask a Question” section of LearnoHub.com
By highlighting the benefits and strategies of effective revision, you can approach your studies more mindfully and achieve better results in your exams. Best of luck bachhon!
Class 11 Chemistry seems to be a quite difficult subject for a lot of students. But, if you get a very good conceptual understanding of the subject, it can be very interesting for you.
We, at LearnoHub, will give our best to make Class 11 Chemistry Redox Reactions NCERT Chapter 7 super-duper easy for you.
We aim at making learning fun as well as engaging for you with our complete end-end learning content with Redox Reactions Class 11 Chemistry Best videos, Notes, NCERT pdf, NCERT complete syllabus, tests and Practice Questions.
Always remember, it is very important to study with full concentration during Revision. Here are a few tips for you on how to revise with full focus:
- Create a Distraction-Free Environment: Find a quiet and comfortable place to study where you can minimize distractions. Turn off or silence your phone, log out of social media accounts, and inform others around you that you need uninterrupted study time. A dedicated study environment will help you focus better.
- Set Specific Goals: Before starting your study session, set clear and achievable goals. Break down your study material into smaller tasks, and plan what you want to accomplish during each session. Having specific goals will give you a sense of direction and purpose, making it easier to concentrate.
- Use the Pomodoro Technique: The Pomodoro Technique is a time management method that involves studying in short, focused intervals, typically 25 minutes, followed by a short break of 5 minutes. After completing four sessions, take a longer break of around 15-30 minutes. This technique can improve focus and productivity by providing regular breaks to recharge.
- Stay Organized: Keep your study materials, notes, and resources well-organized. Having everything you need at hand will save time and reduce distractions caused by searching for materials. Use color-coded folders or digital tools to maintain a structured study system.
- Practice Mindfulness and Meditation: Before you begin studying, take a few minutes to practice mindfulness or meditation. Deep breathing exercises and clearing your mind of distractions can help you approach your study session with a calm and focused mindset.
Remember, studying with full concentration is a skill that takes time and practice to develop. If you find your mind wandering during study sessions, gently bring your focus back to the task at hand and be patient with yourself. With consistent effort, you can improve your ability to concentrate and make the most of your study time.
Last but not the least, To get the best hold on Class 11 Chemistry Redox Reactions Book Chapter 7. Do not forget to check out:
- Redox Reactions Class 11 Chemistry Best videos
- Redox Reactions Class 11 Chemistry NCERT Solutions
- Class 11 Chemistry Redox Reactions Revision notes
- Redox Reactions Class 11 Chemistry DPPS, Download PDF of solutions
- Class 11 Chemistry Redox Reactions Online Tests
- Class 11 Chemistry Sample papers