JEE Chemistry
Structure of Atom


Question 1.
The Second emission line in the atomic spectrum of hydrogen is the Balmer series appears at: [Level: Moderate]
(a) cm-1
(b) cm-1
(c) cm-1
(d) cm-1
Question 2.
Uncertainty in position of a minute particle of mass 40 g in space is 10-7 m. What is the uncertainty in its velocity (in ms-1) (h = 6.6 × 10-34 Js)
[Level: Moderate]
(a) 3.1 x 10-24
(b) 4.7 x 1024
(c) 1.3 x 10-26
(d) 1.89 x 10-19
Question 3.
The graph is plotted between the probability density Ѱ2(r) as a function of distance ‘r’. this graph represent –[Level: Easy]
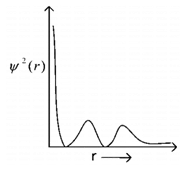
(a) 2s
(b) 3p
(c) 3s
(d) 1s
Question 4.
13.6eV is needed for ionization of a hydrogen atom. An electron in a hydrogen atom in its ground state absorbs 2.25 times as much energy as the minimum required for it to escape from the atom. What is the wavelength of the emitted electron? [Level: Difficult]
(me = 9.1 x 10-31kg, e = 1.602 x 10-19C, h = 6.6 x 10-34J.s)
(a) 4.7 x 10-10m
(b) 47 x 10-11m
(c) 9.1 x 10-10m
(d) 2.9 x 10-10m
Question 5.
Last line of Pfund series for H-atom has wavelength λ1A0 and 2nd line of Paschen series has wavelength λ2A0 then – [Level: Difficult]
(a)
(b)
(c)
(d)
Question 6.
The magnetic momentum of Fe3+ and Cr2+are – [Level: Easy]
(Fe = 26, Cr = 24)
(a)
(b)
(c)
(d)
Question 6.
The Correct set of quantum number is – [Level: Easy]
(a)
(b)
(c)
(d)
Question 7.
Calculate the wavelength in A0of the photon that is emitted when an electron in bohr orbit n = 3 returns to the orbit n = 1 in the hydrogen atom. The ionization potential of the ground state of hydrogen atom is -2.17 x 10-11 erg/ atom. [Level: Moderate]
(a) 1030 A0
(b) 103.0 A0
(c) 1.030 A0
(d) 103 A0
Question 8.
The de Broglie wavelength (λ) associated with a photoelectron varies with the frequency (v) of the incident radiation as, [v0 is threshold frequency] : [Level: Moderate]
(a) λ ∝
(b) λ ∝
(c) λ ∝
(d) λ ∝
Question 9.
This Schrodinger’s wave equation represents – [Level: Moderate]
Where M =
(a) 2s
(b) 3d
(c) 4s
(d) 3p
Question 10.
Choose the correct statement.
(a) The lower the value of value of (n+l) for an orbital, the higher is its energy
(b) If two orbitals have same value of (n+l), the orbital with higher value of n
will have lower energy
(c) The energy of a electron in a multi-electron atom depends on quantum
number n only
(d) The energy of an electron in hydrogen atom depends on quantum
number n only [Level: Easy]
Question 11.
What is the angular velocity(ω) of an electron occupying second orbit of Li2+ ion is – [Level: Moderate]
(a) K2
(b) K2
(c) K2
(d) K2
Question 12.
The orientation of an atomic orbital is governed by [Level: Easy]
(a) Magnetic quantum number
(b) Principal quantum number
(c) Azimuthal quantum number
(d) Spin quantum number
Question 13.
Select the correct curve(s) – [Level: Easy]
Question 14.
Calculate the total number of nodes in 4d orbital [Level: Moderate]
(a) 3
(b) 4
(c) 1
(d) Zero
Question 15.
Correct graphical representation for the following is/are –[Level: Difficult]
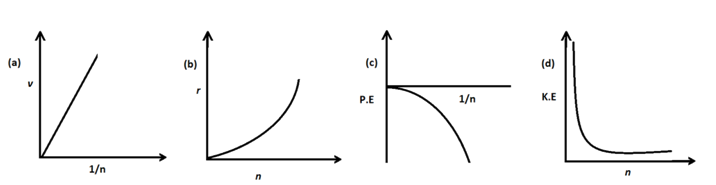
Question 16.
For n = 3, the correct set of l,m are : [Level: Easy]
(a) l = 0, m = 0
(c) l = 3, m = -3,-2,-1,0,1,2,3
(d) l = 1, m = -2,-1,0,1,2
Question 17.
The value of e/m for electron was found to be – [Level: Easy]
(a) 1.75 x 1011 Ckg-1
(b) 1.75 x 108 Cg-1
(c) 1.75 x 105 Cmg-1
(d) All of the above
Question 18.
Given rn+1 - rn-1 = 2rn, where rn ,rn+1 ,rn-1 are Bohr radius for hydrogen atom in nth (n-1)th and (n+1)th shell respectively. Calculate the value of n [Level: Moderate]
Question 19.
A moving electron has 2.5 x 10-18J of kinetic energy. What is the de-broglie wavelength? [Level: Difficult]
(a) 9.8 x 10-28m
(b) 19.8 x 10-12m
(c) 9.8 x 10-28m
(d) 0.98 x 10-28m
Question 20.
Calculate the momentum of a moving particle which has a de Broglie wavelength of 275 pm [Level: Moderate]
(a) 1.24 x 10-24kgms-1
(b) 2.4 x 1024kgms-1
(c) 2.4 x 10-24kgms-1
(d) 2.4 x 10-22kgms-1
**********
In summary, problem-solving after learning a theoretical concept on CBSE Structure of Atom JEE Chemistry is an essential part of the learning process. It enhances your understanding, critical thinking abilities, and retention of knowledge. Moreover, it equips you with valuable skills that are applicable in academic, personal, and professional contexts.
You must have heard of the phrase “Practice makes a man perfect”. Well, not just a man, practice indeed enhances perfection of every individual.
Practicing questions plays a pivotal role in achieving excellence in exams. Just as the adage goes, "Practice makes perfect," dedicating time to solve a diverse range of exam-related questions yields manifold benefits. Firstly, practicing questions allows students to familiarize themselves with the exam format and types of problems they might encounter. This familiarity instills confidence, reducing anxiety and improving performance on the actual exam day. Secondly, continuous practice sharpens problem-solving skills and enhances critical thinking, enabling students to approach complex problems with clarity and efficiency. Thirdly, it aids in identifying weak areas, allowing students to focus their efforts on improving specific topics. Moreover, practice aids in memory retention, as active engagement with the material reinforces learning. Regular practice also hones time management skills, ensuring that students can allocate appropriate time to each question during the exam. Overall, practicing questions not only boosts exam performance but also instills a deeper understanding of the subject matter, fostering a holistic and effective learning experience.
All About Daily Practice Problems on JEE Chemistry Structure of Atom NCERT Chapter 2
Our Daily Practice Problems (DPPs) offer a diverse range of question types, including Multiple Choice Questions (MCQs) as well as short and long answer types. These questions are categorized into Easy, Moderate, and Difficult levels, allowing students to gradually progress and challenge themselves accordingly. Additionally, comprehensive solutions are provided for each question, available for download in PDF format - Download pdf solutions as well as Download pdf Questions. This approach fosters a holistic learning experience, catering to different learning styles, promoting self-assessment, and improving problem-solving skills. With our well-structured DPPs, students can excel in exams while gaining a deeper understanding of the subject matter. Hope you found the content on JEE Chemistry Structure of Atom NCERT Chapter 2 useful.
Last but not least, to get the best hold on JEE Chemistry Structure of Atom NCERT Chapter 2, do not forget to check out: