ICSE 9 Chemistry
Chemical Changes and Reactions


Question 1:
Which among the following is a chemical change?
(a) Steam is converted to water
(b) Dissolution of salt in water
(c) Liquefying oxygen gas
(d) Combustion of methane [Level: Easy]
Question 2:
Precipitation reaction is type of
(a) Combination reaction
(b) Double displacement reaction
(d) Combustion reaction [Level: Easy]
Question 3:
The chemical change involving zinc and aqueous copper sulphate illustrates which of the following reaction?
(a) Combination
(b) Double displacement
(c) Single displacement
(d) Combustion [Level: Moderate]
Question 4:
Give an example of reaction involving change of state. [Level: Easy]
Question 5:
Balance the below given reaction & state the type of reaction:
H2O2 à H2O + O2 [Level: Easy]
Question 6:

Above given reaction is an example of
I. Synthesis
II. Decomposition
III. Electrochemical
IV. Displacement
(a) only I
(d) both II & III [Level: Moderate]
Question 7:
Exothermic reactions can continue without external supply of energy. Justify with the help of suitable chemical reaction. [Level: Difficult]
Question 8:
Selvi was working in lab. She took NH3 gas & HCl gas and mixed them. To her surprise she got only single solid product NH4Cl. Which type of reaction did she carried out?
(a) Combustion
(b) Displacement
(c) Double displacement
(d) Synthesis [Level: Moderate]
Question 9:
Complete and balance the following reactions:
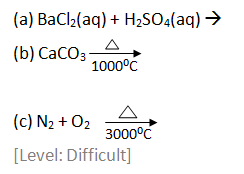
Question 10:
Which is the most reactive element among H & Mg in this equation:
Mg + H2SO4 à MgSO4 + H2 [Level: Moderate]
Question 11:
Give an example of reaction where both evolution of heat & light is involved. [Level: Moderate]
Question 12:
Thermal decomposition means decomposition caused due to
(b) light
(d) catalyst [Level: Easy]
Question 13:
Rameshwar took blue coloured copper sulphate solution & dipped iron nail in it. He observed brown deposit on iron nail & colour of the solution changing to light green. Can you tell what is this light green coloured solution?
(a) FeCO3
(b) CuSO4
(d) CuCO3 [Level: Difficult]
Question 14:
Give an example of endothermic reaction having S as one of the reactants. [Level: Moderate]
Question 15:
Give an example of decomposition reaction in which products formed are
(a) 2 different elements
(b) an element & a compound [Level: Moderate]
Question 16:
NaHCO3 + HCl à NaCl + H2O + CO2
Above given reaction is combination of which 2 types of reaction?
(a) Double displacement & synthesis
(b) Decomposition & Double decomposition
(c) Combination & Displacement
(d) Displacement & Double displacement [Level: Difficult]
Question 17:
The reaction:
NaOH(aq) + HCl(aq) à NaCl(aq) + H2O(l) is
(a) displacement reaction
(b) neutralisation reaction
(c) decomposition reaction
(d) combination reaction [Level: Moderate]
Question 18:
Give an example of combination reaction in which
(a) 2 different elements combine
(b) an element & a compound combines [Level: Moderate]
Question 19:
Raju has some quicklime with him which he was going to use to white wash the walls of his house. When he was about to pour water in quicklime his father asks him to use goggles & hand gloves. Why? [Level: Difficult]
Question 20:
The reaction:
AgNO3(aq) + NaCl(aq) à AgCl(s) + NaNO3(aq) is
(a) displacement reaction
(b) neutralisation reaction
(c) decomposition reaction
(d) double displacement reaction [Level: Moderate]
**********
In summary, problem-solving after learning a theoretical concept on CBSE Chemical Changes and Reactions ICSE 9 Chemistry is an essential part of the learning process. It enhances your understanding, critical thinking abilities, and retention of knowledge. Moreover, it equips you with valuable skills that are applicable in academic, personal, and professional contexts.
You must have heard of the phrase “Practice makes a man perfect”. Well, not just a man, practice indeed enhances perfection of every individual.
Practicing questions plays a pivotal role in achieving excellence in exams. Just as the adage goes, "Practice makes perfect," dedicating time to solve a diverse range of exam-related questions yields manifold benefits. Firstly, practicing questions allows students to familiarize themselves with the exam format and types of problems they might encounter. This familiarity instills confidence, reducing anxiety and improving performance on the actual exam day. Secondly, continuous practice sharpens problem-solving skills and enhances critical thinking, enabling students to approach complex problems with clarity and efficiency. Thirdly, it aids in identifying weak areas, allowing students to focus their efforts on improving specific topics. Moreover, practice aids in memory retention, as active engagement with the material reinforces learning. Regular practice also hones time management skills, ensuring that students can allocate appropriate time to each question during the exam. Overall, practicing questions not only boosts exam performance but also instills a deeper understanding of the subject matter, fostering a holistic and effective learning experience.
All About Daily Practice Problems on ICSE 9 Chemistry Chemical Changes and Reactions NCERT Chapter 2
Our Daily Practice Problems (DPPs) offer a diverse range of question types, including Multiple Choice Questions (MCQs) as well as short and long answer types. These questions are categorized into Easy, Moderate, and Difficult levels, allowing students to gradually progress and challenge themselves accordingly. Additionally, comprehensive solutions are provided for each question, available for download in PDF format - Download pdf solutions as well as Download pdf Questions. This approach fosters a holistic learning experience, catering to different learning styles, promoting self-assessment, and improving problem-solving skills. With our well-structured DPPs, students can excel in exams while gaining a deeper understanding of the subject matter. Hope you found the content on ICSE 9 Chemistry Chemical Changes and Reactions NCERT Chapter 2 useful.
Last but not least, to get the best hold on ICSE 9 Chemistry Chemical Changes and Reactions NCERT Chapter 2, do not forget to check out: