ICSE 8 Chemistry
Elements, Compounds and Mixtures


Question 1:
Which among the following is solid-liquid heterogeneous mixture?
(d) ammonium chloride + water [Level: Moderate]
Question 2:
Virat took a mixture having 3 components. He carried out paper chromatography to separate components. After, drying the filter paper he observed 3 spots as shown in the diagram given below.
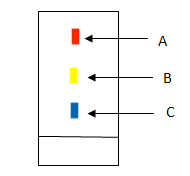
Select the correct statement from below given statements:
(a) Component B is less soluble in mobile phase than component C
(b) Component B is more soluble in mobile phase than component C
(c) Component A is less soluble in mobile phase than component C
(d) Component A is less soluble in mobile phase than component B [Level: Difficult]
Question 3:
Give one word answer for the following:
The solid particles which remain on the filter paper after filtration. [Level: Easy]
Question 4:
You are working in the lab & you want to separate liquid-liquid homogeneous mixture having 2 components A & B. A has boiling point 60⁰C whereas B has boiling point 98⁰C. Which of the following technique you can use to separate the two liquids?
(a) Only Distillation
(b) Evaporation
(c) Both distillation & fractional distillation
(d) Only Fractional distillation [Level: Difficult]
Question 5:
Given below are some statements related to mixtures. Select the correct statement.
(a) A mixture contains particles of two or more pure substances which may be present in it in definite ratio.
(b) Composition of mixture is fixed.
(c) While the formation of mixture, particles of pure substances do not combine chemically.
(d) Original properties of pure substances from which mixture is formed are lost in the mixture. [Level: Difficult]
Question 6:
Gopal categorised some elements given to him into metals, non-metals & metalloids as you can see below in the table. But there are some mistakes committed by him while categorisation. Find out the mistakes & rewrite the table having correct categorisation of elements.
|
Non-metal |
Metalloid |
|
|
Sodium, Mercury, Zinc, Silicon, Nitrogen, Germanium |
Hydrogen, Oxygen, Fluorine, Iron, Sulphur, Potassium |
Boron, Calcium Antimony, Tellurium, Chlorine, Arsenic |
[Level: Difficult]
Question 7:
Give any 3 applications of chromatography. [Level: Moderate]
Question 8:
Give one word answer for the following:
The process by which 2 miscible liquids are separated. [Level: Easy]
Question 9:
Give below is experimental setup for carrying out distillation. Label the inlet & outlet of Liebig condenser. [Level: Moderate]
Question 10:
Which among the following is liquid-liquid homogeneous mixture?
(a) oil + water
(b) kerosene + water
(c) alcohol + water
(d) carbon tetrachloride + water [Level: Moderate]
Question 11:
Which among the following is symbol of Mercury?
(a) M
(b) Me
(d) Hg [Level: Moderate]
Question 12:
Which among the following can be used as stationary phase in paper chromatography?
(a) water
(b) alcohol
(c) filter paper/Whatman filter paper
(d) acetone [Level: Easy]
Question 13:
In which of the following technique, soluble solid is only recovered from homogeneous solid-liquid mixture?
(a) Solvent extraction method
(b) Fractional distillation
(c) Distillation
(d) Evaporation [Level: Moderate]
Question 14:
Which among the following is separation technique used to separate salt from ammonium chloride?
(a) Evaporation
(c) Magnetic separation
(d) Distillation [Level: Easy]
Question 15:
You are given a mixture of water & carbon tetrachloride. Which of the following technique you would use to separate them?
(a) By using separating funnel
(b) Magnetic separation
(c) Sublimation
(d) Filtration [Level: Moderate]
Question 16:
Molecular formula of glucose is
(a) C6H11O6
(c) C12H22O11
(d) C6H11O7 [Level: Moderate]
Question 17:
Composition is fixed in case of
(a) only compounds
(b) mixtures
(d) both compounds & elements [Level: Easy]
Question 18:
In which of the following technique, both solid (soluble) & liquid is recovered from homogeneous solid-liquid mixture?
(a) Sublimation
(b) By using separating funnel
(c) Distillation
(d) Evaporation [Level: Moderate]
Question 19:
Sachin took a mixture of iron filings & sulphur. Accidently he added salt to this mixture. How will you help Sachin to separate the mixture? [Level: Difficult]
Question 20:
Give 3 points of difference between mixtures & compounds. [Level: Moderate]
**********
In summary, problem-solving after learning a theoretical concept on CBSE Elements, Compounds and Mixtures ICSE 8 Chemistry is an essential part of the learning process. It enhances your understanding, critical thinking abilities, and retention of knowledge. Moreover, it equips you with valuable skills that are applicable in academic, personal, and professional contexts.
You must have heard of the phrase “Practice makes a man perfect”. Well, not just a man, practice indeed enhances perfection of every individual.
Practicing questions plays a pivotal role in achieving excellence in exams. Just as the adage goes, "Practice makes perfect," dedicating time to solve a diverse range of exam-related questions yields manifold benefits. Firstly, practicing questions allows students to familiarize themselves with the exam format and types of problems they might encounter. This familiarity instills confidence, reducing anxiety and improving performance on the actual exam day. Secondly, continuous practice sharpens problem-solving skills and enhances critical thinking, enabling students to approach complex problems with clarity and efficiency. Thirdly, it aids in identifying weak areas, allowing students to focus their efforts on improving specific topics. Moreover, practice aids in memory retention, as active engagement with the material reinforces learning. Regular practice also hones time management skills, ensuring that students can allocate appropriate time to each question during the exam. Overall, practicing questions not only boosts exam performance but also instills a deeper understanding of the subject matter, fostering a holistic and effective learning experience.
All About Daily Practice Problems on ICSE 8 Chemistry Elements, Compounds and Mixtures NCERT Chapter 3
Our Daily Practice Problems (DPPs) offer a diverse range of question types, including Multiple Choice Questions (MCQs) as well as short and long answer types. These questions are categorized into Easy, Moderate, and Difficult levels, allowing students to gradually progress and challenge themselves accordingly. Additionally, comprehensive solutions are provided for each question, available for download in PDF format - Download pdf solutions as well as Download pdf Questions. This approach fosters a holistic learning experience, catering to different learning styles, promoting self-assessment, and improving problem-solving skills. With our well-structured DPPs, students can excel in exams while gaining a deeper understanding of the subject matter. Hope you found the content on ICSE 8 Chemistry Elements, Compounds and Mixtures NCERT Chapter 3 useful.
Last but not least, to get the best hold on ICSE 8 Chemistry Elements, Compounds and Mixtures NCERT Chapter 3, do not forget to check out: