Class 12 Chemistry
Chemical Kinetics


Question1.
Calculate the energy or activation from rate constant when two temperature of any reaction is given.
Question2.
What do you understand by half life? Give relation between half life and rate constant of a first order reaction.
Question3.
A radioactive nuclide is produced at a constant rate of α per second. It’s decay constant is λ. If N0 be the no. nuclei at time = 0, then max no. of nuclei possible are :
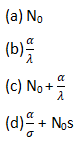
Question4.
A radioactive element undergoing decay is left 30% of is initial weight after certain period of time t. how many period should escape from the start for 45% of element to be left over.
Question5.
An analysis of the rock shows that the relative number of Sr87 and Rb87 ( t1/2 = 4.7 × 10-10 year) atoms is 0.05. what is the age of rock? Assume all the Sr87 have been formed from Rb87 only.
Question6.
Explain following terms –
(a) Order of reaction
(b) Molecularity of a reaction.
Question7.
Select the correct statements -
(a) The rate law of the elementary reaction-
2Aà B + C , Must be r = k[A]2
(b) The rate law for the complex reaction-
A + B à C Might not be r = k[A][B]
(c) If the partial order differ from the stoichiometric coefficient in the balanced reaction, the reaction must be elementary.
(d) If the partial order differ from the stoichiometric coefficient in the balanced reaction, the reaction must be complex.
Question8.
The rate of reaction is depend upon -
(a) temperature
(b) pressure
(c) extent of reaction
(d) initial concentration
Question9.
Rate of reaction is given by –
Rate = k[A]2[B]
What are the units of the rate and rate constant of reaction ?
Question10.
Size of nucleus was obtained by the equation r = R0A1/3, where r is the radius of nucleus of mass no. A and R0 is constant whose value is equal to 1.5 × 10-15 metre.
(Given- 1 amu = 1.66 × 10-24g). Calculate the density of nucleus of mass number A?
Question11.
Write rate reaction for –
N2O5(g) à NO2(g) + 12 O2(g)
O2(g)
Question12.
Identify the correct statements.
In the following reaction
Aà B + C
Initial pressure was found to be 450mm of Hg and it changed to 1200mm of Pb after 15min then-
(a) Half life for A is 4.75min
(b) Rate constant is 0.146 min-1
(c) Partial pressure of C at 30min is 450mm of Pb
(d) Total pressure after 30min is 1100mm of Pb
Question13.
Identify the following graph
(a) First order reaction
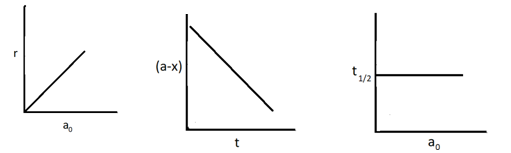
(b) Zero order reaction
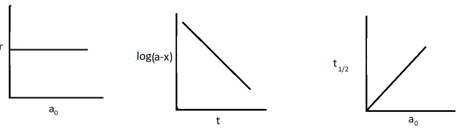
(c) Second order reaction
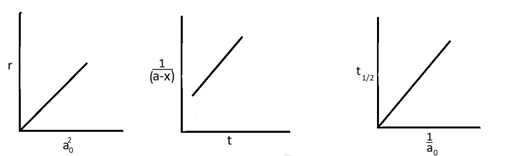
(d) All of the above
Question14.
Give an equation for nth order reaction.
Question15.
What is effective collisions and collision frequency?
Question16.
A(aq) à 2B(aq) + 3C(aq) is a first order reaction.
Time t ∞
Mole or reagent n1 n2
Reaction progress is measured with the help of titration of reagent R. if all A,B and C react with reagent and have n factors 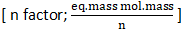 in the ratio of 1:2:3 with the reagent, Calculate the k in term of t, n1 and n2.
in the ratio of 1:2:3 with the reagent, Calculate the k in term of t, n1 and n2.
Question17.
According to Ludwig Boltzmann and James Clark Maxwell, the distribution of kinetic energy may be described by plotting –
a) The fraction of molecules vs kinetic energy.
(b) Reaction coordinate and potential energy.
(c) Concentration and temperature.
(d) Time and Concentration
Question18.
For the reaction, 2A+B⟶3C+D. Which of the following does not express the reaction rate?
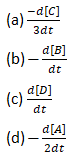
Question19.
Half life period of a first order reaction is 3618 seconds. The specific rate constant of the reaction is –
(a) 1.9 × 10-4 s
(b) 11.9 × 10-2s
(c) 1.9 × 104s
(d) 9.1 × 104s
Question20.
Match the following units –

**********
In summary, problem-solving after learning a theoretical concept on CBSE Chemical Kinetics Class 12 Chemistry is an essential part of the learning process. It enhances your understanding, critical thinking abilities, and retention of knowledge. Moreover, it equips you with valuable skills that are applicable in academic, personal, and professional contexts.
You must have heard of the phrase “Practice makes a man perfect”. Well, not just a man, practice indeed enhances perfection of every individual.
Practicing questions plays a pivotal role in achieving excellence in exams. Just as the adage goes, "Practice makes perfect," dedicating time to solve a diverse range of exam-related questions yields manifold benefits. Firstly, practicing questions allows students to familiarize themselves with the exam format and types of problems they might encounter. This familiarity instills confidence, reducing anxiety and improving performance on the actual exam day. Secondly, continuous practice sharpens problem-solving skills and enhances critical thinking, enabling students to approach complex problems with clarity and efficiency. Thirdly, it aids in identifying weak areas, allowing students to focus their efforts on improving specific topics. Moreover, practice aids in memory retention, as active engagement with the material reinforces learning. Regular practice also hones time management skills, ensuring that students can allocate appropriate time to each question during the exam. Overall, practicing questions not only boosts exam performance but also instills a deeper understanding of the subject matter, fostering a holistic and effective learning experience.
All About Daily Practice Problems on Class 12 Chemistry Chemical Kinetics NCERT Chapter 3
Our Daily Practice Problems (DPPs) offer a diverse range of question types, including Multiple Choice Questions (MCQs) as well as short and long answer types. These questions are categorized into Easy, Moderate, and Difficult levels, allowing students to gradually progress and challenge themselves accordingly. Additionally, comprehensive solutions are provided for each question, available for download in PDF format - Download pdf solutions as well as Download pdf Questions. This approach fosters a holistic learning experience, catering to different learning styles, promoting self-assessment, and improving problem-solving skills. With our well-structured DPPs, students can excel in exams while gaining a deeper understanding of the subject matter. Hope you found the content on Class 12 Chemistry Chemical Kinetics NCERT Chapter 3 useful.
Last but not least, to get the best hold on Class 12 Chemistry Chemical Kinetics NCERT Chapter 3, do not forget to check out: