Class 12 Chemistry
Solutions


Question1.
Calculate (a) molarity (b) molality and (c) mole fraction of KI if the density of 17% (mass/mass) aqueous KI is 1.6gml-1.
Question2.
The freezing point order of a solution of glucose is –
(a) 1% > 3% > 10% > 2%
(b) 1% > 2% > 3% >10%
(c) 1% > 3% > 10% > 2%
(d) 10%> 3% > 2% > 1%
Question3.
H2S, a toxic gas with rotten egg like smell, is used for qualitative analysis. If the solubility of H2S in water at STP is 0.19m, calculate Henry’s law constant.
Question4.
Give relation between molarity and mole fraction.
Question5.
Calculate the mass of Blue vitriol (CuSO4.2H2O) that should be dissolved to prepare 100ml of 0.75 M Aqueous solution. (MB = 159.6g).
Question6.
What concentration of nitrogen should be present in a glass of water at room temperature? Assume temeprature of 250C, a total pressure of 1 ATM and mole fraction of nitrogen in air of 0.78 [ KH For nitrogen = 8.42 x 10-7 M/mm Hg ]
Question7.
1.2% solution of NaCl is isotonic with 7.2% solution of cane sugar. Calculate Van’t Hoff factor of NaCl.
Question8.
Define the following terms:
(i) Ideal solutions
(ii) Azeotrope
(iii) Reverse osmosis
Question9.
The concentration of NO2 in M.P is 11 ppm by volume. Calculate the volume of NO2 per litre of the atmospheric air.
Question10.
Addition of 1.3g of a solute B to 169 g of water raises its boiling point to 100.250C at 1 atm. Compute MB when Kb ( water) is 0.52 K kg mol-1.
Question11.
The osmotic pressure of solution containing 0.10 g of haemoglobin in 10 ml of solution is 2.67 torr at 274 K. Find the molar mass of haemoglobin.
Question12.
Match the following:
(a) Molal elevation constant (kb) - JK-1Mol-1
(b) Roult’s law - MolKg-1
(c) Van off factor - mmHg
(d) Gas constant - KKg-1Mol-1
(e) Molality - Unit less
Question13.
What is van’t Hoff factor? How does it help in the determination of degree of association or dissociation of solute in a solution?
Question14.
What is positive deviation and negative deviation with graphical representation?
Question15.
Vapour pressure of water at room temperature is 23.8mm of hg. What will be the vapour pressure of aqueous solution of sucrose with mole fraction equal to 0.1?
Question16.
What happens when we placed blood in water (hypertonic solution)? Give reason.
Question17.
Which of the following statement is false?
(a) Two different solution of sucrose of same molality prepared in different solvent will have the same depression in freezing
(b) The osmotic pressure of a solution is given by the equation 𝛑 = CRT ( where C is the molarity of the solution)
(c) Decreasing order of osmotic pressure for 0.01M aqueous solution of barium chloride, potassium chloride, acetic acid and sucrose is,
BaCl2 > KCl > CH3COOH > Sucrose
(d) According to Raoult’s law, the vapour pressure exerted by a volatile component of a solution is directly proportional to its mole fraction in the solution.
uestion18.
For a solvent (A) Kf = 31.8 K Kg mol-1 and Kb 5.03 K Kg mol-1. When 3g of a non-volatile solute B is dissolved in 100g of A, the freezing Point is lowered by 3.8 K.
Compute:
(a0 Molar mass of solute (Mb)
(b) Elevation of boling point (∆Tb)
(c) Relative lowering of vapour pressure 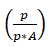
(d) Osmotic pressure π at 300K
Take density of solvent 1.60 g cm-3 and its molar mass (Kb) = 0.154
Question19.
Why does the azeotropic mixture distil without any change in composition?
Question20.
Which of the following fluoride is used as rat poison?
(a) CaF2
(b) KF
(c) NaF
(d) MgF2
**********
In summary, problem-solving after learning a theoretical concept on CBSE Solutions Class 12 Chemistry is an essential part of the learning process. It enhances your understanding, critical thinking abilities, and retention of knowledge. Moreover, it equips you with valuable skills that are applicable in academic, personal, and professional contexts.
You must have heard of the phrase “Practice makes a man perfect”. Well, not just a man, practice indeed enhances perfection of every individual.
Practicing questions plays a pivotal role in achieving excellence in exams. Just as the adage goes, "Practice makes perfect," dedicating time to solve a diverse range of exam-related questions yields manifold benefits. Firstly, practicing questions allows students to familiarize themselves with the exam format and types of problems they might encounter. This familiarity instills confidence, reducing anxiety and improving performance on the actual exam day. Secondly, continuous practice sharpens problem-solving skills and enhances critical thinking, enabling students to approach complex problems with clarity and efficiency. Thirdly, it aids in identifying weak areas, allowing students to focus their efforts on improving specific topics. Moreover, practice aids in memory retention, as active engagement with the material reinforces learning. Regular practice also hones time management skills, ensuring that students can allocate appropriate time to each question during the exam. Overall, practicing questions not only boosts exam performance but also instills a deeper understanding of the subject matter, fostering a holistic and effective learning experience.
All About Daily Practice Problems on Class 12 Chemistry Solutions NCERT Chapter 1
Our Daily Practice Problems (DPPs) offer a diverse range of question types, including Multiple Choice Questions (MCQs) as well as short and long answer types. These questions are categorized into Easy, Moderate, and Difficult levels, allowing students to gradually progress and challenge themselves accordingly. Additionally, comprehensive solutions are provided for each question, available for download in PDF format - Download pdf solutions as well as Download pdf Questions. This approach fosters a holistic learning experience, catering to different learning styles, promoting self-assessment, and improving problem-solving skills. With our well-structured DPPs, students can excel in exams while gaining a deeper understanding of the subject matter. Hope you found the content on Class 12 Chemistry Solutions NCERT Chapter 1 useful.
Last but not least, to get the best hold on Class 12 Chemistry Solutions NCERT Chapter 1, do not forget to check out: