Class 12 Chemistry
Electrochemistry


Question1.
The molar conductivity of a 1.8M solution of an electrolyte is found to be 103.7Scm2mol-1. Calculate the conductivity of its solutions.
Question2.
Define molar conductance and limiting molar conductivity.
Question3.
What would be the Variation of conductivity and molar conductivity with concentration?
Question4.
A cell reaction would be spontaneous if the cell potential and 𝚫rG are respectively.
(a) Positive and negative
(b) Negative, negative
(c) Zero,zero
(d) Positive, zero
Question5.
When two half- cells of electrode potential of E1 and E2 are combined to form a half cell of electrode potential E3, then
(When n1, n2 and n3 are no. of electrons exchanged in first second and combined half-cells).
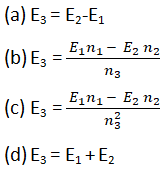
Question6.
What is law of independent migration of ion?
Question7.
A person is performing an experiment of cell potential and got some interesting values of 𝚫G = -193 KJ/mol and n = 2. Calculate the cell potential (in V) for him.
Question8.
State Faraday’s law of electrolysis.
Question9.
How many hours does it take to reduce 3 mol Fe3+ to Fe2+ with 2 ampere current?
(F = 96500 coulomb)
Question10.
What is the role of platinum in hydrogen electrode?
Question11.
The electrical resistance of a thick vertical column of 0.10M KOH solution of diameter 1.5cm and length 75cm is 2.5 × 103 ohm. Calculate its resistivity, conductivity and molar conductivity.
(Given area of Vertical column is 0.922 cm2)
Question12.
For the reaction.
2AgCl(s) + H2(g) à 2Ag(s) + 2H+(0.1M) + 2Cl- (0.1M)
𝚫G0 = -43600J at 250C
Calculate the emf of the cell.
[log10-n = -n]
Question13.
HNO3(aq) is titrated with NaOH conductometrically, graphical representation of titration is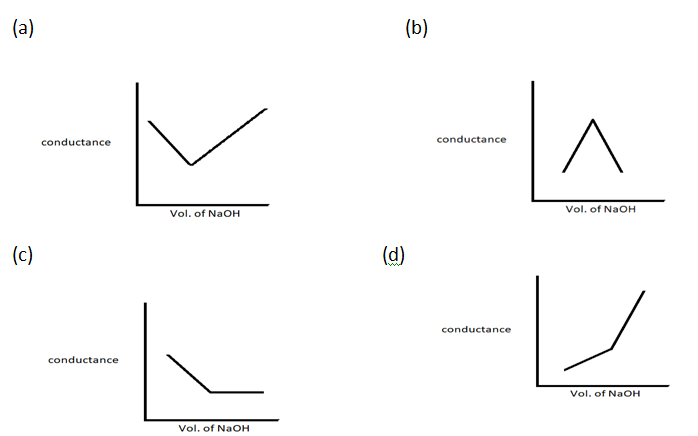
Question14.
A metal that forms self – protecting film of oxide to prevent corrosion is :
(a) Na
(b) Al
(c) Cu
(d) Au
Question15.
Define electrochemical cell. What happens if external potential applied becomes
greater than E0cell of the electrochemical cell.
Question16.
Calculate EMF of the cell,
Zn(s) ӏ Zn2+ (0.01M) ӏӏ Cd2+(0.01M) ӏ Cd(s)
E0Zn2+/zn = - 0.76 V ; E0Cd2+/cd = -0.40V
Question17.
The temperature coefficient of a cell whose operation is based on the reaction.
Calculate the change in entropy ( in J/K mol) during the operation.
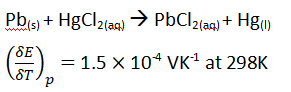
Question18.
Match the following units –
(a) Conductance - Sm-1(equiv L-1)-1
(B) Molar conductance – Sm2mol-1
(c) Resistivity – cm-1
(d) Equivalent conductance – S
(e) Cell constant – Ohm m
Question19.
Silver is electro-deposited on a metallic vessel of total surface area 900cm2 by using a
current of 0.5 ampfor 2 hrs. calculate the thickness of silver deposited. Density of silver =
10.5gcm-3
(Atomic mass of silver =108amu, 1F = 96500C)
Question20.
Which of the following fluoride is used as rat poison?
(a) CaF2
(b) KF
(c) NaF
(d) MgF2
***********
In summary, problem-solving after learning a theoretical concept on CBSE Electrochemistry Class 12 Chemistry is an essential part of the learning process. It enhances your understanding, critical thinking abilities, and retention of knowledge. Moreover, it equips you with valuable skills that are applicable in academic, personal, and professional contexts.
You must have heard of the phrase “Practice makes a man perfect”. Well, not just a man, practice indeed enhances perfection of every individual.
Practicing questions plays a pivotal role in achieving excellence in exams. Just as the adage goes, "Practice makes perfect," dedicating time to solve a diverse range of exam-related questions yields manifold benefits. Firstly, practicing questions allows students to familiarize themselves with the exam format and types of problems they might encounter. This familiarity instills confidence, reducing anxiety and improving performance on the actual exam day. Secondly, continuous practice sharpens problem-solving skills and enhances critical thinking, enabling students to approach complex problems with clarity and efficiency. Thirdly, it aids in identifying weak areas, allowing students to focus their efforts on improving specific topics. Moreover, practice aids in memory retention, as active engagement with the material reinforces learning. Regular practice also hones time management skills, ensuring that students can allocate appropriate time to each question during the exam. Overall, practicing questions not only boosts exam performance but also instills a deeper understanding of the subject matter, fostering a holistic and effective learning experience.
All About Daily Practice Problems on Class 12 Chemistry Electrochemistry NCERT Chapter 2
Our Daily Practice Problems (DPPs) offer a diverse range of question types, including Multiple Choice Questions (MCQs) as well as short and long answer types. These questions are categorized into Easy, Moderate, and Difficult levels, allowing students to gradually progress and challenge themselves accordingly. Additionally, comprehensive solutions are provided for each question, available for download in PDF format - Download pdf solutions as well as Download pdf Questions. This approach fosters a holistic learning experience, catering to different learning styles, promoting self-assessment, and improving problem-solving skills. With our well-structured DPPs, students can excel in exams while gaining a deeper understanding of the subject matter. Hope you found the content on Class 12 Chemistry Electrochemistry NCERT Chapter 2 useful.
Last but not least, to get the best hold on Class 12 Chemistry Electrochemistry NCERT Chapter 2, do not forget to check out: