Class 12 Chemistry
Biomolecules


Question1.
Structure of disaccharide is formed by glucose and fructose is given below. Identify anomeric carbon atoms in monosaccharide units.
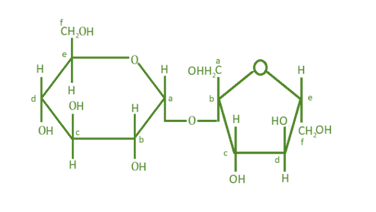
(a) ‘a’ carbon of glucose and ‘a’ carbon of fructose
(b) ‘a’ carbon of glucose and ‘e’ carbon of fructose
(c) ‘a’ carbon of glucose and ‘b’ carbon of fructose
(d)‘f’ carbon of glucose and ‘f’ carbon of fructose
Question2.
Name of the cyclic ring present in fructose.
(a)Pyridine
(b) Pyrole
(c) Furan
(d) Indole
Question3.
What do you understand by anomeric carbon? Explain by giving one example.
Question4.
Name two aldose sugars present in nucleic acid.
Question5.
All amino acid are optically active except –
(a) valine
(b) arginine
(c) glycine
(d) isoleucine
Question6.
Carbohydrate have a general formula of Cx(H2O)y
Both Acetic acid and Rhamnose are belongs to carbohydrate category.
(a) Acetic acid is carbohydrate and Rhamnose is not
(b) Both are carbohydrate
(c) Rhamnose is a carbohydate and Acetic acid is not
(d) Both are not Carbohydrate
Question7.
Lactose is a milk sugar in which glucose and galactose is linked with –
(a) C-1 of Glucose and C-4 of galactose
(b) C-1 of galactose and C-4 of glucose
(c) C-3 fructose and C-2 of Glucose
(d) C-1 of Glucose and C-4 of glucose
Question8.
Protein can be classified into two types in the basis of their molecular shape, i.e. fibrous and globular protein. Example of globular protein –
(a) myosin
(b) insulin
(c) cellulose
(d) amylopectin
Question9.
What do you understand by isoelectric PH?
Question10.
Give name of two conformation test for aldehydic group of Glucose with one reaction?
Question11.
Which of the following reaction of glucose can be explained only by its cyclic structure?
(a) Glucose form pentaacetate.
(b) Glucose reacts with hydroxylamine to form an oxime.
(c) Pentaacetate of glucose does not react with hydroxylamine.
(d) Glucose is oxidised by nitric acid to form gluconic acid.
Question12.
Which of the following is a purine base?
(a) Guanine
(b) Thymine
(c) Thyroxine
(d) Uracil
Question13.
In fibrous protein, polypeptide chains are held together by:
(a) van der waal’s forces
(b) disulphide linkage
(c) electrostatic Forces of attraction
(d) hydrogen bonds
Question14.
Dinucleotide is obtained by joining two nucleotides together by phosphodiester linkage.
etween which carbon atoms of pentose sugar of nucleotide are these linkage present
(a) 5’ and 3’
(b) 1’ and 5’
(c) 5’ and 5
(d) 3’ and 3’
Assertion and reasons
(a) If (A) and (R) are correct statement and (R) is the correct explanation of the (A).
(b) If both (A) and (R) are not correct.
(c) If (A) is incorrect statement but (R) is correct statement
(d) If (A) is correct statement but (R) is incorrect statement
(e) If both (A) and (R) are correct statement but (R) is not the correct explanations of
(A)
Question15.
A. Vitamin D can be stored in our body.
B. Vitamin D is fat soluble vitamin.
Question16.
The major groves and minor groves in DNA is –
(a) 22 Å and 12 Å wider Respectively
(b) 12 Å and 22 Å wider Respectively
(c) 30 Å and 5 Å wider Respectively
(d) 15 Å and 15 Å wider Respectively
Question17.
The 3-D model of DNA is given by –
(a) Watson and Crick
(b) Friedrich Miescher
(c) Leslie Orgel
(d) Gerardus Johannes Mulder
Question18.
Vitamin containing cobalt metal is
(a) Vitamin D
(b) Vitamin B6
(c) Vitamin B12
(d) Vitamin C
Question19.
What do you understand by first and second class protein?
Question20.
Match the following enzymes with the reaction they catalyse.
(a) Invertase – Decomposition of urea into NH3 and CO2
(b) Maltase – Conversion of glucose into ethyl alcohol
(c) Pepsin – Hydrolysis of maltose into glucose
(d) Urease – Hydrolysis of cane sugar
(e) Zymase – Hydrolysis of protein into peptides.
***********
In summary, problem-solving after learning a theoretical concept on CBSE Biomolecules Class 12 Chemistry is an essential part of the learning process. It enhances your understanding, critical thinking abilities, and retention of knowledge. Moreover, it equips you with valuable skills that are applicable in academic, personal, and professional contexts.
You must have heard of the phrase “Practice makes a man perfect”. Well, not just a man, practice indeed enhances perfection of every individual.
Practicing questions plays a pivotal role in achieving excellence in exams. Just as the adage goes, "Practice makes perfect," dedicating time to solve a diverse range of exam-related questions yields manifold benefits. Firstly, practicing questions allows students to familiarize themselves with the exam format and types of problems they might encounter. This familiarity instills confidence, reducing anxiety and improving performance on the actual exam day. Secondly, continuous practice sharpens problem-solving skills and enhances critical thinking, enabling students to approach complex problems with clarity and efficiency. Thirdly, it aids in identifying weak areas, allowing students to focus their efforts on improving specific topics. Moreover, practice aids in memory retention, as active engagement with the material reinforces learning. Regular practice also hones time management skills, ensuring that students can allocate appropriate time to each question during the exam. Overall, practicing questions not only boosts exam performance but also instills a deeper understanding of the subject matter, fostering a holistic and effective learning experience.
All About Daily Practice Problems on Class 12 Chemistry Biomolecules NCERT Chapter 10
Our Daily Practice Problems (DPPs) offer a diverse range of question types, including Multiple Choice Questions (MCQs) as well as short and long answer types. These questions are categorized into Easy, Moderate, and Difficult levels, allowing students to gradually progress and challenge themselves accordingly. Additionally, comprehensive solutions are provided for each question, available for download in PDF format - Download pdf solutions as well as Download pdf Questions. This approach fosters a holistic learning experience, catering to different learning styles, promoting self-assessment, and improving problem-solving skills. With our well-structured DPPs, students can excel in exams while gaining a deeper understanding of the subject matter. Hope you found the content on Class 12 Chemistry Biomolecules NCERT Chapter 10 useful.
Last but not least, to get the best hold on Class 12 Chemistry Biomolecules NCERT Chapter 10, do not forget to check out: