Class 10 Science
Acids, Bases and Salts


Question1.
Acid and bases indicator are mixture of –
(a) their salt
(b) dyes
(c) solid indicator
(d) basics salt
Question2.
Sodium and potassium are two metal, their carbonates reacts with strong acid they produces __________.
Question3.
You have three solution A, B and C. The pH of Solution A is 3 ,pH of solution B is 7.5 and pH of solution C is 13. Which solution has more H+ ion and OH-? Which of the solution is acidic and basic in nature?
Question4.
Why 10 ml acetic acid cannot neutralize the 10ml NaOH while only 8ml of hydrochloric acid neutralized the 10ml NaOH?
Question5.
Give the name of the process which involve Production of sodium hydroxide from sodium chloride –
(a) Solvay process
(b) Chlor-alkali process
(c) Brine degradation
(d) None of these
Question6.
(a) Identify the A and B.
(b) Write chemical name of B.
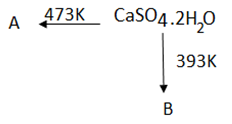
Question7.
What happens when excess carbon dioxide is passed through lime water? Give its reaction.
Question8.
To determine the presence of oxalic acid ,NaOH is used for assay can you determine the neutral value of solution,
If 40ml of oxalic acid is used to neutralize 75ml of NaOH. How much oxalic acid is required to neutralize 10ml of NaOH?
Question9.
Mixing an acid or base with water results in decrease in the concentration of ions –
(a) H3O+/H+
(b) H3O+/OH-
(c) NaOH /HCl
(d) H2O/H+
Question10.
What is the role of antacid in indigestion?
Question11.
What is acid rain and pH of acid rain? How it affects the aquatic life?
Question12.
What is water of crystallization?
Question13.
The acid used for the manufacture of fertilizers and explosives is
(a) Nitric acid
(b) Sulfuric acid
(c) Phosphoric acid
(d) Hydrochloric acid
Question14.
What if ferric oxide is dipped in a HCl filled beaker? Name the product formed.
Question15.
Tomato is a natural source of ____________ acid.
(a) Ascorbic acid
(b) Citric acid
(c) Tartaric acid
(d) Oxalic acid
Question16.
Sodium carbonate is a basic salt because it is a salt of a -
(a) Strong acid and weak base
(b) Weak base and weak acid
(c) weak acid and strong base
(d) strong acid and strong base
Question17.
Alkalis are
(a) acid, which are soluble in water
(b) acid, which are insoluble in water
(c) base, which are insoluble in water
(d) base, which are soluble in water
Question18.
Give two property and chemical name of following –
(a) Baking soda
(b) Gypsum
(c) Washing soda
(d) Bleaching powder
Question19.
Enamel of made up of ________ and get corrode when pH of mouth is below ________.
Question20.
The concentration of hydrogen ion is a sample of lemon drink is 2 x 10-5M . What is its pH?
**********
In summary, problem-solving after learning a theoretical concept on CBSE Acids, Bases and Salts Class 10 Science is an essential part of the learning process. It enhances your understanding, critical thinking abilities, and retention of knowledge. Moreover, it equips you with valuable skills that are applicable in academic, personal, and professional contexts.
You must have heard of the phrase “Practice makes a man perfect”. Well, not just a man, practice indeed enhances perfection of every individual.
Practicing questions plays a pivotal role in achieving excellence in exams. Just as the adage goes, "Practice makes perfect," dedicating time to solve a diverse range of exam-related questions yields manifold benefits. Firstly, practicing questions allows students to familiarize themselves with the exam format and types of problems they might encounter. This familiarity instills confidence, reducing anxiety and improving performance on the actual exam day. Secondly, continuous practice sharpens problem-solving skills and enhances critical thinking, enabling students to approach complex problems with clarity and efficiency. Thirdly, it aids in identifying weak areas, allowing students to focus their efforts on improving specific topics. Moreover, practice aids in memory retention, as active engagement with the material reinforces learning. Regular practice also hones time management skills, ensuring that students can allocate appropriate time to each question during the exam. Overall, practicing questions not only boosts exam performance but also instills a deeper understanding of the subject matter, fostering a holistic and effective learning experience.
All About Daily Practice Problems on Class 10 Science Acids, Bases and Salts NCERT Chapter 2
Our Daily Practice Problems (DPPs) offer a diverse range of question types, including Multiple Choice Questions (MCQs) as well as short and long answer types. These questions are categorized into Easy, Moderate, and Difficult levels, allowing students to gradually progress and challenge themselves accordingly. Additionally, comprehensive solutions are provided for each question, available for download in PDF format - Download pdf solutions as well as Download pdf Questions. This approach fosters a holistic learning experience, catering to different learning styles, promoting self-assessment, and improving problem-solving skills. With our well-structured DPPs, students can excel in exams while gaining a deeper understanding of the subject matter. Hope you found the content on Class 10 Science Acids, Bases and Salts NCERT Chapter 2 useful.
Last but not least, to get the best hold on Class 10 Science Acids, Bases and Salts NCERT Chapter 2, do not forget to check out: